BioCapsid Therapeutics’ novel drug would, potentially, provide a therapy for diverse diseases of complex pathophysiology.
In addition to our current focus, treatment of sepsis (https://doi.org/10.18632/oncotarget.27448), we anticipate that our drug would be an efficient treatment of patients suffering from the following diseases:
- Acute kidney injury (AKI) – efficacy demonstrated in a mouse model of mercury-induced AKI (https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0002998).
- Traumatic brain injury (TBI) – preliminary study demonstrated partial recovery from brain damage in mice (unpublished).
- Multidrug-resistant bacteria – the rapid replication rate of microorganisms, coupled with their high mutability and selection pressure, leads to the emergence of resistance to newly developed antibiotics and to bacteriophages.
Acute kidney injury (AKI)
The kidneys maintain the body’s overall fluid balance, while filtering out waste and toxic substances through their tubules. Acute kidney injury is characterized by abrupt kidney dysfunction, caused by sepsis, ischemia or nephrotoxic agents. AKI may lead to long-term morbidity, requiring life-long dialysis or kidney transplantation, both with added complications.
Many patients are at an increased risk for AKI because they either suffer from previous kidney dysfunction or from diabetes, which predisposes to kidney injury. AKI develops in these patients following exposure to a second “Hit” such as contrast agents or nephrotoxic drugs as well as following episodes of reduced blood pressure (shock) and sepsis.
At present there is no successful way to prevent or treat AKI, other than giving symptomatic support. Therefore, the development of a new treatment to prevent AKI, in high-risk patients, or to treat AKI in those who develop it is of great importance.
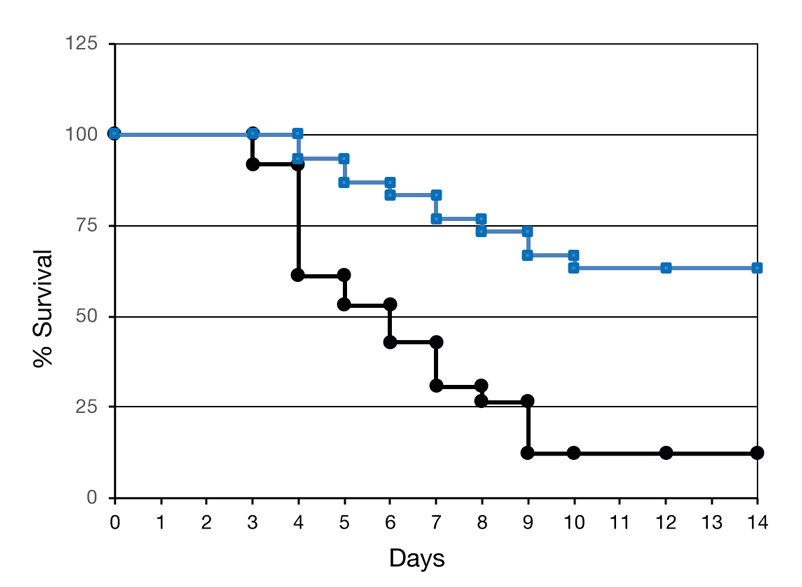
Studies were performed on the potential therapeutic effect of the NCs for AKI treatment (https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0002998) in a mercury-induced mouse model.
NCs (—) or placebo (—) were administered in 3 daily doses, on days -3, -2 and -1.
Mercury was injected into both groups on day 0. The NCs increased survival of the
sick mice over 5 fold, from 12% to over 63%, as seen below.
Additional evidence for the therapeutic effect of the NCs came from pathological examinations. Open tubules are seen in the NC-treated mouse (AKI + NCs), while most of the tubules of the placebo-treated one (AKI + Placebo) were clogged by dead epithelial cells (indicated by white arrows).
AKI + NCsTraumatic brain injury (TBI)
Traumatic brain injury (TBI) is a major cause of long-term morbidity in all injured patients. It leads to prolonged, and even permanent neurological disabilities, and thus to major costs. Developing a drug that will reduce secondary brain injury following head trauma is of vital importance. It is the only way to improve the outcome of the TBI patient.
Preliminary studies demonstrated that the NCs are capable of reducing the severity of neurological dysfunction in a mouse model. TBI was induced using a weight drop device (Beni-Adani et al, J Pharmacol Exp Ther, 2001; 296:57-63). NCs were administered in 3 daily doses, beginning at ~3 hours after the insult (—). The placebo treated mice are seen as (—). Neurological damage is expressed as neurological severity score (NSS), assessed through 10 different parameters.
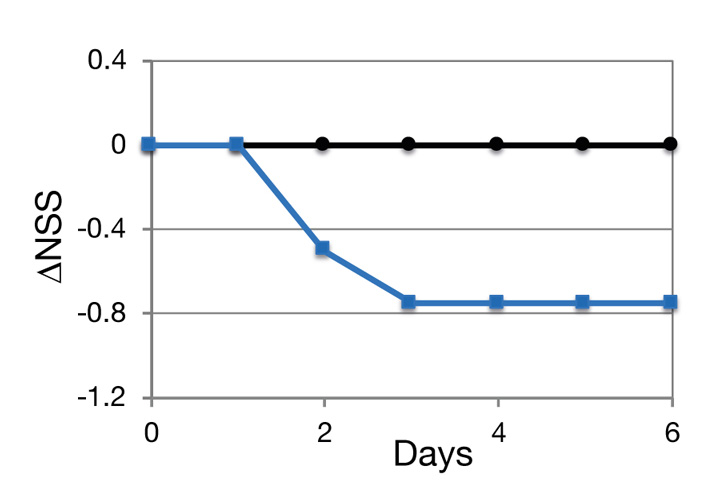
The data indicate that both the second and the third administrations of NCs reduced the neurological severity score, suggesting that additional NC treatments would have further improved the state of the brain.
Multidrug-resistant pathogens
In recent years, less than a century after the discovery of penicillin, we face a sharp increase in drug-resistant bacteria. Antibiotics kill bacteria by blocking bacterial enzymes that are essential for their reproduction. The rapid multiplication of bacteria, coupled with their high rate of mutation, lead to the development of drug-resistant microbes, especially given the strong selection provided by extensive antibiotic treatments. Drug-resistant bacteria are a major medical problem worldwide, particularly in the hospital setting. Designing new antibiotic molecules is presently used to reduce the problem; however, the emergence of resistance to these new drugs is just a question of time. Similarly, killing the bacteria via their natural rivals, the bacteriophages, may also lead to resistance through mutation of the relevant bacteriophage receptor on the bacterial cell wall.
The mechanism by which the NCs fight sepsis is the induction of multiple pathways within the host, rather than affecting the bacteria. Although some of these pathways lead to direct killing of the bacterium, they also protect the host via regulation of the immune response through multiple signaling pathways with overlapping functions. For this reason, it is not anticipated that resistant pathogens will develop following treatment with the NCs.
